Abstract
Introduction: Large B-cell lymphomas (LBCL) are aggressive but curable subtypes of non-Hodgkin lymphoma (NHL). Patients with multiple relapses or refractory (R/R) LBCL have a very poor prognosis with a median overall survival of about 6 months. Until recently, allogenic stem cell transplantation (allo-SCT) was the only curative option for chemotherapy-refractory patients or relapses after an autologous stem cell transplantation (auto-SCT). However, its efficacy was limited by significant toxicity and high non-relapse mortality (NRM), mainly due to graft-versus-host disease (GVH) and infections. Lately, approval of autologous CD19-directed chimeric antigen receptor T-cell therapy (CAR-T) significantly improved therapeutic management for R/R LBCL after 2 or more lines of systemic therapy, providing long-term remissions in 35 to 40% of patients with manageable toxicity. Most recently, ZUMA-7 and TRANSFORM trials demonstrated the superiority of second-line CAR-T over standard of care chemotherapy with or without auto-SCT for R/R LBCL. To investigate the positioning of allo-SCT in the current therapeutic landscape of R/R LBCL, we performed a retrospective analysis of patients treated with allo-SCT or CAR-T for this indication in our institute.
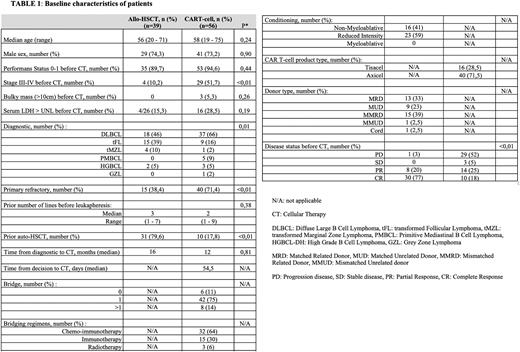
Materiel & Method: All patients who underwent allo-SCT or CAR-T from February 2010 to January 2022 at Institute Paoli-Calmettes (Marseille, France) were included in this retrospective study. LBCL included diffuse large B-cell lymphoma DLBCL, high-grade B-cell lymphoma with translocations involving MYC and BCL2 or BCL6, or Burkitt like-lymphoma (HGBL), Primary Mediastinal B-cell lymphoma (PMBCL), Grey-Zone lymphoma (GZL), and transformed follicular or marginal zone lymphoma (t-FL and t-MZL).
Results: We analyzed 95 successive patients with R/R LBLC treated with allo-SCT (from Feb 2010 to Dec 2020; N = 39; median age: 56 (range 20-71); Donor type: matched related donor: 13; mismatched related donor: 15; matched unrelated doner: 9; mismatched unrelated donor: 1 cord blood = 1) or CAR T cell therapy (from Jan 2019 to Jan 2022; N = 56; median age : 58 (range 19-75); Axi-Cel n = 40, Tisa-Cel n = 16). Median number of prior lines received before cellular therapy were 3 and 2 for allo-SCT and CAR-T groups respectively, bridging therapy excluded. The CAR-T group had a significantly greater proportion of patients with primary refractory disease (71,4% vs 38,4%). The two-year OS and PFS rates for the allo-SCT group were 69% [95% confidence interval (CI): (56-85)] and 66% [95% CI: (53-83)] respectively. The cumulative incidence of NRM at 2-year post allo-SCT was 13% [95% CI: (6-30)]. The two-year OS and PFS rates for the CAR-T group were 67% [95% confidence CI: (53-84)] and 43% [95% CI: (31-61)] respectively. At a median follow-up of 23,2 months, 28 patients experienced relapse/progression after CAR-T. 5 patients underwent allo-SCT after CAR-T failure, 1 of whom was in partial response at the time of conditioning and the 4 others with complete responses. With a median follow up of 14 months: 4 patients are still in response after allo-SCT and only 1 patient had an early relapse (<3 months).
Conclusion: Our results confirm efficacy and safety of CAR-T in R/R LBCL with durable complete responses in about 40% of patients in the 3rd line setting. However, early failures after infusion require prompt diagnosis and management because they portend a very dismal prognosis. In this context, our data show that allo-SCT remains a curative option for selected LBCL patients relapsing after CAR T-cell therapy with manageable toxicity.
Disclosures
Brisou:Incyte: Honoraria; Gilead: Honoraria; BMS: Honoraria; Novartis: Honoraria. Inchiappa:ASTRAZENECA: Honoraria; JANSSEN: Honoraria; ABBVIE: Honoraria; Gilead: Honoraria. Brenot Rossi:CURIUM PHARMA: Honoraria; NOVARTIS AAA: Honoraria. Chabannon:JANSSEN PHARMACEUTICALS: Membership on an entity's Board of Directors or advisory committees; TERUMO BCT: Speakers Bureau; MILTENYI BIOTECH: Research Funding; FRESENIUS KABI: Research Funding; EBMT: Membership on an entity's Board of Directors or advisory committees; BELLICUM PHARMACEUTICALS: Membership on an entity's Board of Directors or advisory committees; GILEAD: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; NOVARTIS: Speakers Bureau; BMS/CELGENE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SANOFI SA: Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal